Request A Quote
Our business is most valuable when it helps our customers. Please fill out the form with as much detail as possible so we can respond with the most helpful solution.
Fluorine is a chemical element, with symbol F and has an atomic number 9. It is the lightest halogen and, under normal circumstances, exists as an extremely dangerous light yellow diatomic gas. It is reactive in nature, due to the fact that it is the most electronegative component. Fluorine forms compounds with all other substances, even some noble gases. All other elements react with fluorine, and no chemical can release fluorine from any of its compounds. As a result, fluorine cannot be found isolated in nature, making its discovery challenging. It is found in warm waters and volcanic gasses. Fluorite is its principal source, but it can also be found in cryolite, seawater, bones, and teeth. Under special circumstances, electrolysis is used to create fluorine.
The most reactive element is fluorine, which reacts quickly with practically all other elements. It is a non-metal halogen and draws electrons to itself due to its high electronegative nature.
So does Fluorine conduct electricity?
Fluorine is a non-metal that does not conduct electricity. Due to its extreme electronegativity and high degree of reactivity, it only takes electrons and does not lose them. And due to this property of fluorine, it restricts the movement of electrons and fails to conduct electricity. Metals conduct heat and electricity better than non-metals. Understanding fluorine’s molecular and physical makeup is crucial to understanding why it cannot carry electricity.
Fluorine is a great nuclear material and an excellent electrical insulator. This makes it ideal for the production of nuclear power plants and electrical towers. Additionally, fluorine is in the manufacturing of semiconductor devices- electronic components that utilize semiconductor material. Both molecular and atomic fluorine fulfills this role and is used for plasma etching and flat panel display production.
SF6, Sulfur Hexafluoride, is commonly used as an electrical insulator in high voltage equipment that transmits and distributes electricity. The U.S electric power industry has used SF6 in circuit breakers, gas-insulated substations, and other switchgear used in the transmission system to manage the high voltages carried between generating stations and customer load centers.
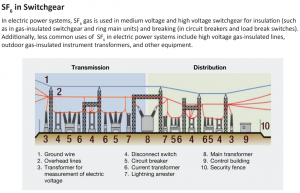
Gas-insulated switchgear in a typical electric power transmission and distribution system. – Figure adapted from https://www.epa.gov/eps-partnership/overview-sf6-emissions-sources-and-reduction-options-electric-power-systems
Despite being the most electronegative element, there are a multitude of electrical applications of which fluorine compounds are useful for. Make sure to check out our electronic gases page to view our products!
Previous Fluorine Chemistry
Our business is most valuable when it helps our customers. Please fill out the form with as much detail as possible so we can respond with the most helpful solution.